“New Blood Biomarker Raises Controversy: Is G2019S Mutation the Key to Parkinson’s?”
Parkinson’s disease biomarkers, G2019S mutation blood test, phospho-RAB12 clinical trials
—————–
Understanding the LRRK2 G2019S Mutation and Its Impact on Parkinson’s Disease
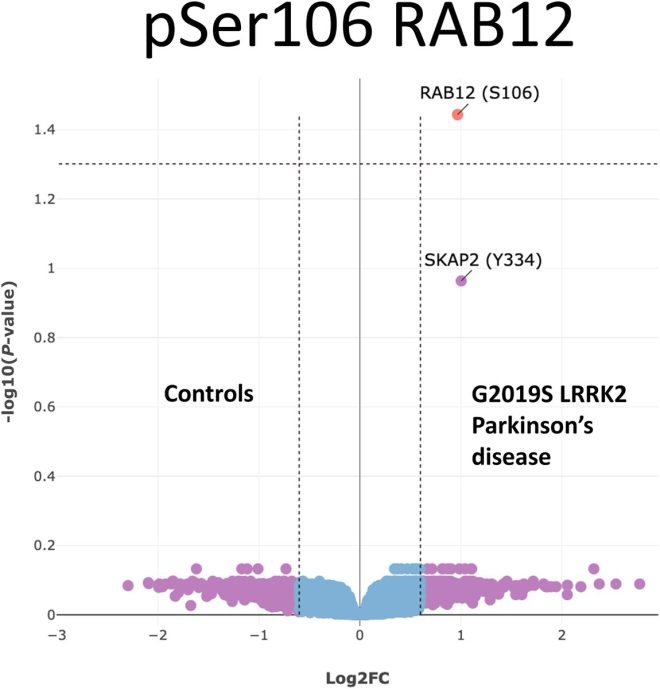
Parkinson’s disease is a progressive neurological disorder that primarily affects movement. Among its various genetic causes, the LRRK2 activating mutation G2019S has emerged as the most prevalent. This mutation plays a significant role in the development of Parkinson’s disease, making it a focal point for research aimed at understanding the underlying mechanisms and potential treatment options. Recent findings by Cortés et al. have identified elevated levels of phospho-RAB12 in the blood of individuals carrying the G2019S mutation, presenting new opportunities for early diagnosis and clinical trial applications.
The Significance of the LRRK2 Gene in Parkinson’s Disease
The LRRK2 gene, or Leucine-Rich Repeat Kinase 2, is vital for various cellular functions, including neuronal signaling and cytoskeletal dynamics. The G2019S mutation in this gene leads to increased kinase activity, which disrupts normal cellular processes and contributes to the degeneration of dopaminergic neurons—a hallmark of Parkinson’s disease. Understanding the implications of this mutation is critical for developing targeted therapies.
Elevated Phospho-RAB12 as a Biomarker
Cortés et al. have made a significant breakthrough by identifying elevated phospho-RAB12 levels in the blood as an endogenous biomarker for individuals with the G2019S mutation. RAB12 is a member of the RAB family of small GTPases, which are pivotal in intracellular vesicle trafficking. The phosphorylation state of RAB12 reflects cellular signaling pathways that are activated in response to the G2019S mutation.
- YOU MAY ALSO LIKE TO WATCH THIS TRENDING STORY ON YOUTUBE. Waverly Hills Hospital's Horror Story: The Most Haunted Room 502
This discovery is particularly important for several reasons:
1. **Early Diagnosis**: The ability to detect elevated phospho-RAB12 levels in blood samples can facilitate earlier diagnosis of Parkinson’s disease in mutation carriers. Early intervention is crucial for managing symptoms and slowing disease progression.
2. **Clinical Trial Utility**: As research progresses toward potential therapies targeting the G2019S mutation, biomarkers like phospho-RAB12 can serve as valuable tools in clinical trials. They can help in stratifying participants, evaluating treatment efficacy, and monitoring disease progression.
3. **Personalized Medicine**: Understanding the biological implications of the G2019S mutation allows for a more personalized approach to treatment. By identifying individuals at risk and monitoring their disease status through biomarkers, healthcare providers can tailor interventions to meet the specific needs of patients.
Implications for Future Research
The identification of phospho-RAB12 as a biomarker opens several avenues for future research. Scientists can now explore the pathways that lead to increased RAB12 phosphorylation in mutation carriers and how these changes contribute to the clinical manifestations of Parkinson’s disease. Moreover, this research can inform the development of new therapeutic strategies aimed at normalizing RAB12 signaling and mitigating the effects of the G2019S mutation.
Additionally, further studies could investigate the potential for phospho-RAB12 levels to correlate with disease severity or progression. Such insights could enhance the clinical utility of this biomarker, making it a cornerstone for monitoring the efficacy of novel treatments.
The Role of Genetic Testing in Parkinson’s Disease Management
As genetic testing becomes more accessible, identifying individuals with the G2019S mutation is increasingly feasible. Genetic counseling can provide individuals and families with vital information about their risk for developing Parkinson’s disease and the implications for family members. Understanding one’s genetic status can empower patients and their families to make informed decisions regarding lifestyle changes, participation in clinical trials, and monitoring for early signs of the disease.
Conclusion
The LRRK2 G2019S mutation represents a significant genetic contributor to Parkinson’s disease. The recent findings by Cortés et al. concerning elevated phospho-RAB12 levels in the blood present an exciting advancement in the field. These elevated levels not only offer a potential biomarker for early diagnosis but also serve as a valuable tool for clinical trials and personalized medicine approaches.
As research continues, it is essential to harness the insights gained from studying the G2019S mutation and associated biomarkers. By advancing our understanding of the biological mechanisms underlying Parkinson’s disease, we can pave the way for innovative therapies that target the root causes of the disorder, ultimately improving the quality of life for those affected by this challenging disease.
In conclusion, the work by Cortés et al. underscores the importance of ongoing research into genetic factors contributing to Parkinson’s disease. The identification of phospho-RAB12 as a biomarker not only enhances our understanding of the G2019S mutation but also holds promise for improving early diagnosis and treatment strategies, offering hope to countless individuals and families affected by Parkinson’s disease.

The LRRK2 activating mutation G2019S is the most frequent genetic cause of Parkinson’s disease. Cortés et al. identify elevated phospho-RAB12 levels in blood as an endogenous biomarker of G2019S mutation carriers, with potential utility in clinical trials. https://t.co/ew89IeptzZ https://t.co/5VrFrvxcAI
The LRRK2 activating mutation G2019S is the most frequent genetic cause of Parkinson’s disease.
Parkinson’s disease is a complex neurological disorder that affects millions worldwide, and understanding its genetic underpinnings is crucial for effective treatment and management. One of the most significant breakthroughs in this field has been the identification of the LRRK2 gene, particularly the activating mutation G2019S. This mutation stands out as the most common genetic cause of Parkinson’s disease, accounting for a substantial number of cases, especially in specific populations.
The LRRK2 gene, when mutated, can lead to increased kinase activity, which disrupts normal cellular functions. Researchers are keenly focused on this mutation, as it not only contributes to our understanding of the disease’s pathology but also opens doors to targeted therapies. Why is this important? Because knowing that G2019S is a significant player in the development of Parkinson’s disease helps scientists and clinicians tailor their approaches to prevention, diagnosis, and treatment.
Cortés et al. identify elevated phospho-RAB12 levels in blood as an endogenous biomarker of G2019S mutation carriers.
In a recent study, Cortés et al. made a compelling discovery: they found elevated levels of phospho-RAB12 in the blood of individuals carrying the G2019S mutation. This finding is groundbreaking because it suggests that phospho-RAB12 could serve as a reliable endogenous biomarker, providing critical insights into the biology of the disease and its progression.
So, what exactly does this mean? Essentially, it means that measuring phospho-RAB12 levels could help identify individuals who carry the G2019S mutation, even before they exhibit any clinical symptoms. This is a game-changer in the field of neurology. Early identification can lead to timely interventions, which could slow disease progression or even enhance the quality of life for those affected.
The implications of this research extend beyond just identification. If phospho-RAB12 levels can indicate disease presence or progression, they could also be instrumental in clinical trials. For instance, researchers could use these biomarkers to evaluate the effectiveness of new therapies. Imagine a world where we could track a treatment’s impact on a patient’s biomarker levels, leading to more personalized and effective treatment plans.
With potential utility in clinical trials.
The potential utility of phospho-RAB12 in clinical trials cannot be overstated. Biomarkers like these are invaluable tools for researchers. They enable the development of more targeted therapies and help in the selection of appropriate patient populations for clinical trials. For people with the G2019S mutation, this means that their treatment plans could become more tailored and effective.
For example, if a new drug is designed to inhibit the activity of LRRK2, measuring phospho-RAB12 levels could provide insights into whether the drug is working as intended. If levels decrease, it could signal that the treatment is having a positive effect on the underlying disease mechanism. This real-time feedback loop could significantly accelerate the drug development process, ensuring that only the most effective therapies make it to market.
Furthermore, the identification of phospho-RAB12 as a biomarker could help in stratifying patients in clinical trials. Researchers could focus on individuals who are more likely to benefit from a particular therapy based on their biomarker status. This stratification not only enhances the chances of trial success but also minimizes exposure to ineffective treatments for patients, making the clinical trial process more ethical and efficient.
Understanding the broader implications of the G2019S mutation.
Understanding the LRRK2 activating mutation G2019S and its role in Parkinson’s disease is just the tip of the iceberg. The broader implications of this mutation extend to how we think about genetic testing and personalized medicine. Genetic testing for LRRK2 mutations can help identify at-risk individuals, allowing for early interventions that could delay or even prevent the onset of symptoms.
Moreover, as research continues to unveil the complexities of Parkinson’s disease, integrating genetic insights into clinical practice will be essential. For instance, if a patient is found to carry the G2019S mutation, healthcare providers can implement tailored monitoring strategies and lifestyle modifications aimed at optimizing brain health.
The ongoing research into LRRK2 mutations also encourages collaboration across various fields, including genetics, neurology, and pharmacology. This interdisciplinary approach is vital for addressing the multifaceted nature of Parkinson’s disease and developing comprehensive treatment strategies.
Future research directions.
As we look to the future, several key research directions emerge from the findings of Cortés et al. First and foremost, further studies are needed to validate phospho-RAB12 as a reliable biomarker across diverse populations. It’s essential to understand how these levels might vary based on ethnicity, age, and other genetic factors.
Additionally, researchers should explore the mechanisms by which the G2019S mutation leads to increased phospho-RAB12 levels. Understanding these pathways could unveil new therapeutic targets, ultimately leading to innovative treatments that tackle the root causes of the disease rather than just alleviating symptoms.
Moreover, the potential for phospho-RAB12 to serve as a predictive biomarker for treatment response in clinical trials warrants extensive investigation. Developing standardized protocols for measuring and interpreting phospho-RAB12 levels will be crucial in ensuring consistent results across studies.
Finally, patient education and awareness about the importance of genetic testing for LRRK2 mutations should be prioritized. Empowering individuals with knowledge about their genetic risk can lead to better health outcomes and foster a proactive approach to managing their health.
In summary, the LRRK2 activating mutation G2019S is not just a genetic marker; it is a critical avenue for understanding and combating Parkinson’s disease. The work by Cortés et al. highlights the promise of phospho-RAB12 as a biomarker, paving the way for future innovations in diagnosis and treatment. As research progresses, it is our hope that these advancements will lead to a brighter future for those affected by this challenging condition.
